High-grade gliomas
Last edited on : 26/09/2024
High-grade gliomas (grades III and IV) are primary malignant tumors of the central nervous system originating from glial cells. They include anaplastic astrocytomas, anaplastic oligodendrogliomas, and glioblastomas. The most common in adults is glioblastoma multiforme (the most aggressive grade IV glioma), with an incidence of approximately 5 cases per 100,000 inhabitants per year.
Despite an extremely low metastatic potential, their vital prognosis unfortunately remains grim in the short to medium term, with a median survival with treatment of 30 months for grade III and 10 to 15 months for grade IV (5-year survival of 5 to 10%).
Histology and Oncogenesis Elements
While the histological diagnosis of high-grade glioma is generally straightforward (glial character confirmed by GFAP antibodies, degree of malignancy evaluated according to standard criteria: number of mitoses, abnormal nuclei, degree of necrosis, endothelial proliferation), their classifications (WHO histological classification or Sainte-Anne histo-radiological classification) remain imprecise and poorly reproducible. Furthermore, some grade III gliomas closely resemble grade II gliomas (traditionally classified as "low-grade" gliomas while they are now also considered true malignant lesions) due to their more favorable clinical evolution and radiological appearance. The opinion of the pathologist should therefore be compared with the results of radiological and isotopic imaging.
In any case, the WHO distinguishes between anaplastic gliomas (grade III: anaplastic astrocytomas, oligodendrogliomas, and oligoastrocytomas) and glioblastomas (grade IV).
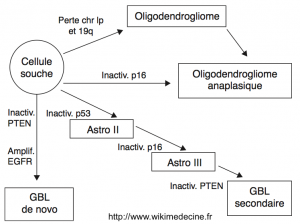
Oncogenesis is characterized by a proliferation of glial cells secondary to the overexpression of proto-oncogenes and the loss of tumor suppressors. Numerous cytogenetic abnormalities have been found (++ deletions of 1p, 9p, 10, 13q, 17p, 19q, 22q and gains on chromosome 7), and their number is correlated with tumor aggressiveness.
The historical hypothesis that glioblastomas only arise de novo has been abandoned, and it is now well established that grade II gliomas inexorably progress to high-grade. Currently, three major pathways of progression from glial cells are described:
- Pathway of grade II astrocytomas → III (anaplastic astrocytoma) → IV (secondary glioblastoma)
- Grade II gliomas inevitably degenerate to grade III and then to grade IV as a result of accumulating genetic alterations characterized by early mutations of p53 and a PDGF/PDGFR autocrine loop. Mutations or deletions of p16 and CDKN2A are found in anaplastic foci. The progression to glioblastoma would be linked to the occurrence of deletions of chromosome 10 and hypermethylation of promoter regions of RB1 and MGMT.
- Pathway of "de novo" glioblastomas = primary
- Their occurrence is characterized by amplification of EGFR, loss of chromosome 10, PTEN mutation, and amplification of MDM2. p53 mutations are rare.
- Pathway of oligodendrogliomas (grade II) → anaplastic oligodendrogliomas
- High frequency of heterozygous deletion of 1p and 19q, PDGF/PDGFR autocrine loop. Anaplastic degeneration would be accompanied by loss of p16/CDKN2A, 10q, RB1, and p53, and overexpression of EGFR.
In the vast majority of cases, there is no family history of brain tumors. Cases occurring within the framework of hereditary tumor syndromes (Li-Fraumeni syndrome, Lynch syndrome, etc.) are the exception.
Various occupational risk factors (rubber industry, lead, pesticides) have been implicated, although no direct causal link has been demonstrated. However, as with meningiomas, cases of radiation-induced high-grade gliomas are well established.
Clinic
Clinical manifestations may include:
- Due to the lesion itself (infiltration, compression, vascular bias)
- Seizures (20 to 50% of patients)
- Focal deficits varying according to location and progressive onset
- Headaches (stretching of vascular or meningeal elements)
- Due to intracranial hypertension (HTIC)
- Headaches (++ morning, bilateral, stabbing, worsening with position changes or Valsalva maneuver), nausea, vomiting, rarely decreased visual acuity due to papilledema (more frequent in children)
- Systemic repercussions
- Unexplained mechanism (metabolic and/or immune disorders? functional?)
- Asthenia, decreased libido, anxiety
Although they can occur at any age, anaplastic astrocytomas preferentially occur between ages 25 and 34, while de novo glioblastomas preferentially occur between ages 65 and 74.
They generally present with rapidly progressive focal neurological deficits and/or recurrent seizures.
Except for disseminations within the brain and spinal parenchyma or cerebrospinal fluid (CSF) (meningeal gliomatosis, occurring in 4 to 20% of cases), the metastatic potential of these tumors is extremely low. However, cases of bone or pleural metastases have been described.
Complementary Examinations
Brain CT scan without and with contrast
It can reveal calcifications, hemorrhagic changes, a mass effect,… The lesion and edema generally appear as a hypodense area. Contrast uptake indicates a breach of the blood-brain barrier (BBB). Differential diagnosis can sometimes be difficult with a stroke, encephalitis, abscess, or metastasis.
Brain MRI with gadolinium (Gold Standard)
In grade III astrocytomas, a diffuse hyposignal is generally observed in T1 (lesion + edema), areas enhancing with gadolinium corresponding mainly to zones of anaplastic transformation, a diffuse hypersignal in FLAIR and T2.
In glioblastomas, a large very heterogeneous hypointense lesion in T1 is typically observed, often taking contrast heterogeneously (+ sometimes annular contrast uptake) and hyperintense in T2, with multiple hemorrhagic changes and a large perilesional edema. Unlike lymphomas and abscesses, necrotic areas are typically frequent (hypersignals T2 and FLAIR), perfusion is increased (neovascularization), and there is generally no diffusion restriction (sensitivity and specificity of 90%).
Sometimes smaller expansive lesions at a distance are observed (multicentric gliomas), a vast lesion sheet involving several lobes or even the entire brain (cerebral gliomatosis), or pachymeningitis (meningeal gliomatosis).
Magnetic resonance spectroscopy is useful in difficult radiological diagnoses and to specify the degree of malignancy of the tumor.
Lumbar Puncture
Its role is very limited here. It may be of interest in cases of pachymeningitis → the anatomic-pathology of the cerebrospinal fluid can assist in differential diagnosis (meningeal gliomatosis, meningeal sarcomatosis, metastatic pachymeningitis, sarcoidosis, chronic infections). However, it is often contraindicated due to the mass effect of the tumor (risk of engagement due to decompensation of intracranial hypertension).
Anatomic Pathology: Stereotactic Biopsy or Surgical Specimen
An anatomic-pathological confirmation is essential for the definitive diagnosis. It can be based on a biopsy or directly on the surgical excision product if an operative indication is initially retained. In addition to diagnostic confirmation, it also allows for molecular and histochemical analyses characterizing the histology and genetics of the tumor to better guide treatments.
Brain PET Scan
- With Methionine
- ++ to determine the degree of malignancy of the tumor
- With FDG
- ++ to guide a stereotactic biopsy (hot spot), useful for differential diagnosis between recurrence and radionecrosis, may be useful for differential diagnosis with lymphoma.
MIBI SPECT-CT Brain
Currently primarily used for the differential diagnosis between recurrence and radionecrosis.
Miscellaneous
Systematic neuropsychological evaluation +- ophthalmological. Discuss the need for additional examinations based on the established differential diagnosis.
Prognostic Factors
Generally, poor prognostic factors (faster progression) include: age > 45 years, multiform glioblastoma, incomplete surgical resection based on radiology, low performance index.
The grim prognosis of high-grade gliomas should be tempered by the fact that significant survival prolongation can be obtained in some rare cases: some grade III gliomas progress similarly to grade II (up to several decades, with radiological appearance being the best predictive factor) and very superficial tumors may allow for optimal iterative surgical resections.
Quality of Surgical Resection
The benefit in terms of quality of life depends on each case according to clinical expression, location, and iatrogenic risks. Total macroscopic resection alone has shown no benefit on lifespan. However, a limited benefit has been demonstrated (++ 8 to 14 weeks) in cases of optimal resection based on MRI.
The benefit is sometimes greater for superficial lesions allowing for easy iterative resections → automatic surgery is absurd and always should be discussed on a case-by-case basis. If applicable, prefer resection of lesions based on macroscopic + radiological findings.
Therapeutic Principles - Treatments
The approach is purely palliative; the expected benefit in terms of survival must be carefully weighed against the repercussions on quality of life. Each case should be discussed, but the most common initial management combines surgery, radiotherapy, and chemotherapy.
Surgery
Surgery remains the Gold Standard. However, achieving a "cure" is illusory: "recurrence" is inevitable regardless of the quality of resection. Three objectives are possible:
- Diagnostic: diagnostic anatomic pathology via biopsy or excision – generally essential for determining the subsequent treatment and definitively ruling out a potentially curable differential diagnosis.
- Increase survival and/or relieve symptoms: reduction of tumor volume, evacuation of cystic content, release or diversion of cerebrospinal fluid.
- Potentiate the effect of adjuvant treatments: resection of poorly vascularized areas that are more resistant to chemotherapy and radiotherapy.
Total resection based on intraoperative radiology is preferred. However, the reduced lifespan benefit must be weighed against potential postoperative complications depending on location. Therefore, any intervention should be carefully discussed.
Radiotherapy
Increases the median survival of all brain neoplasms (overall, the survival gain is estimated at 3 months for high-grade gliomas). Conventional adjuvant radiotherapy to surgery remains the Gold Standard. Gamma knife can be discussed on a case-by-case basis.
In the weeks following, a transient aggravation may be observed (edema). Corticosteroids may then be useful. After more than a month, neurological deterioration may occur (demyelination), generally transient and without possible treatment. After more than 4 months, radionecrosis, cerebral atrophy, or disorders of the hypothalamic-pituitary axis may be encountered. Post-radiation encephalopathies are usually too late to be encountered in such a context.
Chemotherapy
Increases the median survival by approximately 2 months. No demonstrated difference between oral, IV, or local intraoperative chemotherapy. No consensus on the timing to initiate it (as soon as possible? saved for recurrences?). The molecules that have demonstrated efficacy are: BCNU, CCNU, fotemustine, cisplatin, carboplatin, procarbazine, etoposide, cyclophosphamide, hydroxyurea, bleomycin, temozolomide (++ in first-line). Carmustine implants may also be used.
No consensus on protocols, but here’s one that has demonstrated its effectiveness: temozolomide PO 75 mg/m²/day 1 hour before each irradiation during radiotherapy, adjuvant treatment one month post-radiotherapy of 150 mg/m²/day for 5 days every 28 days (200 mg/m²/day starting from the second cycle). Anti-pneumocystis prophylaxis should be ensured during treatment (e.g., trimethoprim/sulfamethoxazole 800/160 mg 3 times/week). The duration of chemotherapy is not standardized (usually 6 cycles).
Miscellaneous
Various approaches, not yet validated, are under evaluation: anti-angiogenic agents, agents modifying auto and paracrine loops, immunomodulators, alternative electromagnetic fields.
In emergencies, and in cases of significant tumor edema, corticosteroids may have a spectacular transient effect (! to be avoided as much as possible until the differential diagnosis of lymphoma is ruled out).
An antiepileptic treatment should be permanently initiated in case of a seizure occurrence. However, there is no indication for prophylactic treatment (although an antiepileptic treatment is sometimes temporarily instituted [1 to 3 months] postoperatively... there is no evidence supporting this approach). Regarding glioblastomas, the potentiation of temozolomide’s effect by valproate has been suggested for several years... without any demonstrated benefit in terms of survival.
Bibliography
Batchelor T, Initial postoperative therapy for glioblastoma and anaplastic astrocytoma, UpToDate, 2018
Batchelor T et al., Management of recurrent high grade gliomas, UpToDate, 2018
Bradley WG et al., Neurology in clinical practice, 5th ed., Butterworth-Heinemann, e-dition, 2007
Chatel M et al., Gliomes de haut grade, Encyclopédie Médico-Chirurgicale, Neurologie, Elsevier, Paris, 2005
Dietrich J et al., Clinical presentation, initial surgical approach, and prognosis of high grade gliomas, UpToDate, 2018
Osborn AG, Diagnostic imaging : brain, Amirsys, USA, 2d ed., 2009
Shih HA, Radiation therapy for high grade gliomas, UpToDate, 2018





